Water Splitting
Water splitting technology is characterized by a process that uses a chemical reaction to separate water (H2O) into oxygen and hydrogen. Effective water splitting can be achieved using a number of different techniques including electrolysis, photosynthesis, photoeclectrochemical, photocatalytic, radiolysis, photobiological, and thermolysis. Highly conformal ALD has proven critical to optimize cell efficiencies as latest achievements have focused on the use of nanoparticles and thin film catalysts to split water at lower reaction temperatures. Economical and efficient water splitting is a critical component for hydrogen generation as an alternative energy source. Research in this field will investigate and test the feasibility of transitioning to a hydrogen economy.
Water Splitting Technologies: Clearing a Path to Clean Hydrogen
Clean hydrogen plays a big role in the shift to sustainable energy, and water splitting makes it possible through electrolysis. To make the process efficient and scalable, engineers rely on thin-film deposition and surface engineering to optimize the catalysts and electrodes that make it all work.Factors for Precision Separation
Tools like atomic layer deposition (ALD) help build high-surface-area catalyst films with just the right structure and composition, while carefully engineered surfaces improve electron transfer and keep electrodes stable under stress. And with smart system designs, clean gas separation can happen with minimal energy loss.Engineers Use These Tools To:
- Boost hydrogen output with optimized catalyst layers
- Increase electrode durability with coatings that last
- Move from R&D to industrial-scale hydrogen production
Veeco: Repeatable Success at Every Layer
From ALD and (physical vapor deposition) PVD systems to electrode coating platforms, 91��Ƭ��gives engineers the control they need to fine-tune every layer. With built-in diagnostics and reliable process tools, performance stays consistent, making it easier to scale clean hydrogen, one reaction at a time.
Photo-oxidation of water
Atomic layer deposition techniques can be used to create high surface area structures for wafer splitting applications. The fabrication of high surface area conducting and transparent frameworks were developed for the photo-oxidation of water (water splitting).
Atomic Layer Deposition (ALD) films using the Savannah® S200 were deposited using the Exposure mode technique for ITO and Fe2O3 on inverse opal structures, to generate a high surface area nanostructure.
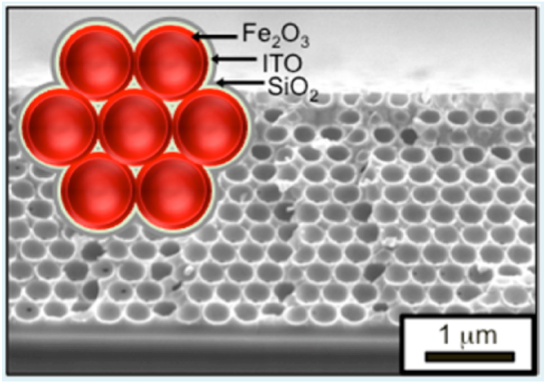
High specific surface area transparent and conducting frameworks for photo-oxidation of water. Iron oxide Fe2O3, ITO and SiO2 are deposited by ALD on an inverse scaffold structure. Ref: Riha, S. C, et al.. Acs Appl Mater Inter 5, 360–367 (2013).
Films
- Indium-tin oxide (ITO) using InCp, TDMASn, and O3
- Ferrocene (Fe2O3) using FeCp2 and O3
Results
- Highly transparent, large surface area nanolaminate stacks were created for water splitting using ALD techniques.
- The onset of water oxidation was shifted by -200 mV and the photocurrent at 1.53 V vs. the reversible hydrogen electrode was tripled. (compared with flat photo-anodes).
Greenlight water oxidation via Ti substitution in Fe2O3
Ti alloying was used to improve ultrathin (6 nm thick) hematite conversion efficiencies, in particular the hole collection efficiency generated by green photons ( 500 – 600 nm). The Savannah® S200 was used to deposit films of both TiO2 and Fe2O3 in this study.
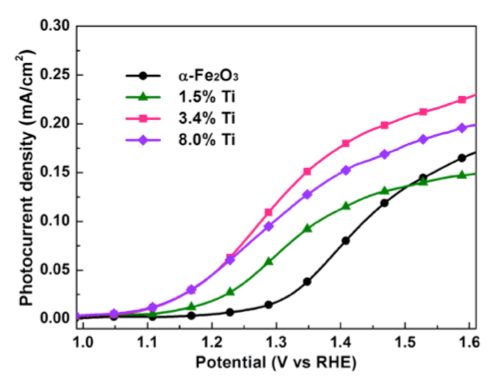
Titanium alloying of Fe2O3 is used to increase to improve catalytic utilization for photoelectrochemical oxidation of waterRef: Kim, D. W. et al. Greenlighting Photoelectrochemical Oxidation of Water by Iron Oxide. ACS Nano 141203161851003 (2014).
Results
- Ti substitution in ultrathin Fe2O3 increases the lifetime of surface-localized holes.
- Increased photocurrent performance was observed with the Ti-substituted films.
- Enhanced absorbed photon-to-current efficiency (APCE) was observed, particularly in the 500 – 600 nm range.
- No change was observed in optical absorption.
REFERENCES – Recent publications done on 91��Ƭ��CNT ALD platforms
- Dias, P. et al. Transparent Cuprous Oxide Photocathode Enabling a Stacked Tandem Cell for Unbiased Water Splitting. Adv. Energy Mater. n/a–n/a (2015). doi:10.1002/aenm.201501537
- Luo, J. et al. Targeting Ideal Dual‐Absorber Tandem Water Splitting Using Perovskite Photovoltaics and CuInxGa1‐xSe2 Photocathodes. Adv. Energy Mater. (2015). doi:10.1002/aenm.201501520
- Landsmann, S. et al. Design Guidelines for High-Performance Particle-Based Photoanodes for Water Splitting: Lanthanum Titanium Oxynitride as a Model. ChemSusChem n/a–n/a (2015). doi:10.1002/cssc.201500830
- Barreca, D. et al. Fe2O3–TiO2 Nano‐heterostructure Photoanodes for Highly Efficient Solar Water Oxidation. Advanced Materials Interfaces n/a–n/a (2015). doi:10.1002/admi.201500313
- Michaux, K. E. et al. Visible Photoelectrochemical Water Splitting Based on a Ru(II) Polypyridyl Chromophore and Iridium Oxide Nanoparticle Catalyst. The Journal of … (2015). doi:10.1021/acs.jpcc.5b05711
- Luo, J. et al. Solution Transformation of Cu2O into CuInS2 for Solar Water Splitting. Nano Lett 15, 1395–1402 (2015).
- Schoen, D. T., Atre, A. C., García-Etxarri, A., Dionne, J. A. & Brongersma, M. L. Probing Complex Reflection Coefficients in One-Dimensional Surface Plasmon Polariton Waveguides and Cavities Using STEM EELS. Nano Lett 15, 1–8 (2014).
- Kim, D. W. et al. Greenlighting Photoelectrochemical Oxidation of Water by Iron Oxide. ACS Nano 141203161851003 (2014). doi:10.1021/nn503869n
- Azevedo, J. et al. On the stability enhancement of cuprous oxide water splitting photocathodes by low temperature steam annealing. Energy Environ. Sci. 7, 4044–4052 (2014).
- Yang, X. et al. Improving Hematite-based Photoelectrochemical Water Splitting with Ultrathin TiO 2by Atomic Layer Deposition. Acs Appl Mater Inter 140804065734002 (2014). doi:10.1021/am500948t
- Klahr, B. & Hamann, T. Water Oxidation on Hematite Photoelectrodes: Insight into the Nature of Surface States through In Situ Spectroelectrochemistry. J. Phys. Chem. C 118, 10393–10399 (2014).
- Zandi, O., Beardslee, J. A. & Hamann, T. Substrate Dependent Water Splitting with Ultrathin α-Fe 2O 3Electrodes. J. Phys. Chem. C 140501145249004 (2014). doi:10.1021/jp4116657
- Tilley, D. S. & Grätzel, M. Ruthenium Oxide Hydrogen Evolution Catalysis on Composite Cuprous Oxide Water-Splitting Photocathodes. Advanced Functional … 1–9 (2014). doi:10.1002/adfm201301106
- Yang, X., Du, C., Liu, R., Xie, J. & Wang, D. Balancing photovoltage generation and charge-transfer enhancement for catalyst-decorated photoelectrochemical water splitting: A case study of the hematite/MnOx combination. Journal of Catalysis 304, 86–91 (2013).
- Liu, M., Nam, C.-Y., Black, C. T., Kamcev, J. & Zhang, L. Enhancing Water Splitting Activity and Chemical Stability of Zinc Oxide Nanowire Photoanodes with Ultrathin Titania Shells. J. Phys. Chem. C 130610143036005 (2013). doi:10.1021/jp404032p
- Riha, S. C. et al. Atomic Layer Deposition of a Sub-Monolayer Catalyst for the Enhanced Photoelectrochemical Performance of Water Oxidation with Hematite. ACS Nano 130212010712000 (2013). doi:10.1021/nn305639z
- Riha, S. C., DeVries Vermeer, M. J., Pellin, M. J., Hupp, J. T. & Martinson, A. B. F. Hematite-based Photo-oxidation of Water Using Transparent Distributed Current Collectors. Acs Appl Mater Inter 5, 360–367 (2013).
- Klug, J. A. et al. Low temperature atomic layer deposition of highly photoactive hematite using iron(iii) chloride and water. J. Mater. Chem. A 1, 11607 (2013).
- Maijenburg, A. W. et al. Electrochemical synthesis of coaxial TiO2-Ag nanowires and their application in photocatalytic water splitting. J. Mater. Chem. A (2013). doi:10.1039/c3ta14551d
- Klahr, B., Gimenez, S., Fabregat-Santiago, F., Bisquert, J. & Hamann, T. W. Photoelectrochemical and Impedance Spectroscopic Investigation of Water Oxidation with ‘Co–Pi’-Coated Hematite Electrodes. J Am Chem Soc 134, 16693–16700 (2012).
- Paracchino, A. et al. Ultrathin films on copper(I) oxide water splitting photocathodes: a study on performance and stability. Energy & Environmental Science 5, 8673–8681 (2012).
- Klahr, B., Gimenez, S., Fabregat-Santiago, F., Bisquert, J. & Hamann, T. W. Electrochemical and photoelectrochemical investigation of water oxidation with hematite electrodes. Energy & Environmental Science 5, 7626–7636 (2012).
- Klahr, B. M., Martinson, A. B. F. & Hamann, T. W. Photoelectrochemical Investigation of Ultrathin Film Iron Oxide Solar Cells Prepared by Atomic Layer Deposition. Langmuir 27, 461–468 (2011).
- Liu, R. et al. Water Splitting by Tungsten Oxide Prepared by Atomic Layer Deposition and Decorated with an Oxygen-Evolving Catalyst. Angew. Chem. Int. Ed. 50, 499–502 (2010).